1. What's the main advantanges about your factory?
We have a professional product research and development team,the most advanced production line and detecting instruments, sales team with humanistic service(including after-sale service).Conforms to GMP standand and meets USP35,ChP2015,EP9,JP17.
2. What’s the quality assurance you provided and how do you control quality?
•Established a procedure to check products at all stages of the manufacturing process-raw materials, process materials, validated or tested materials finished goods etc.. Besides, we have also developed a procedure which identifies the inspection and test status of all stages of the manufacturing process.
•100% inspection in assembly lines. All controls, inspection, equipment, fixtures, total production resources and skills are inspected to ensure they consistently achieve the required quality levels. our product is under the supervision of QA and QC,firmly conforms to GMP standard.
3. Can I know your MOQ and delivery time?
Our Minium Order Quantity is 1Kg, products can be sent within one week after payment confirmed.
4. Can purchaser come to audit?
We warmly welcome customers' audit,it's an effective way to build a better business relationship.
5. Advantages:
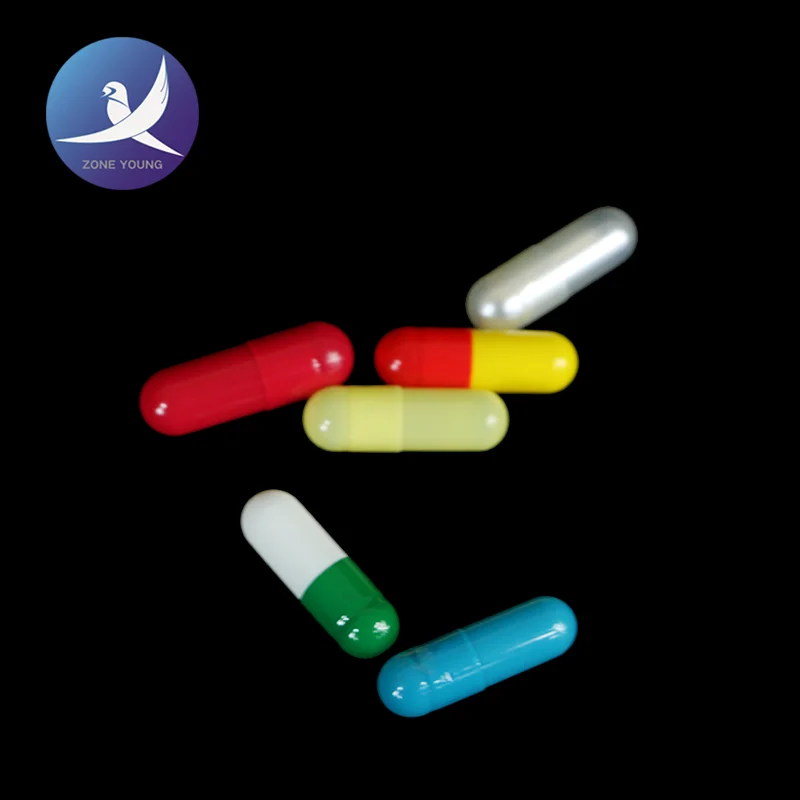
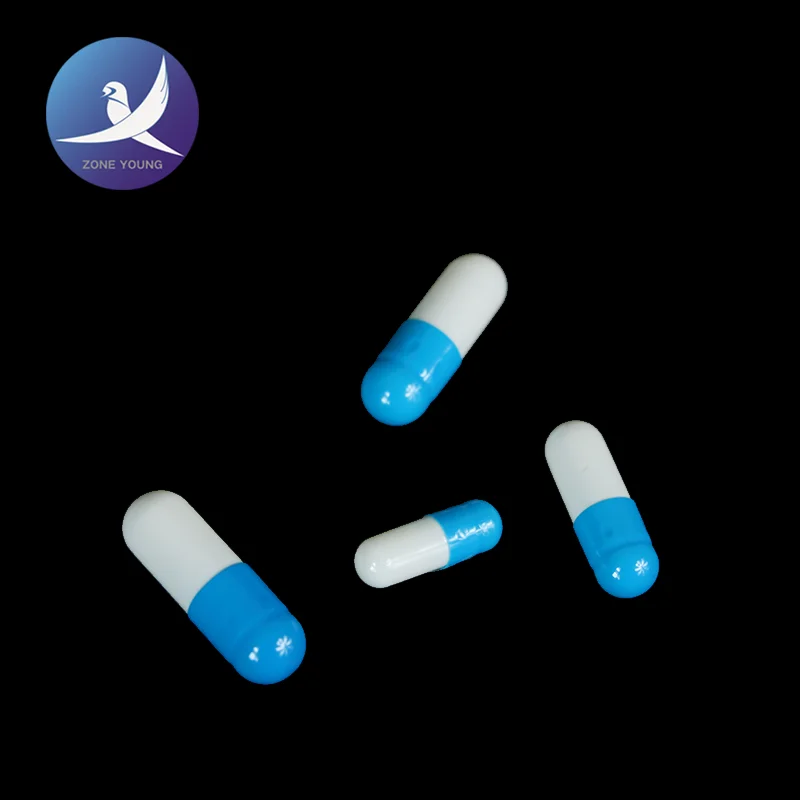
Gelatin empty capsules are adding an appropriate amount of pigment from the gelatin capsule, opacifiers made products into transparent, translucent and opaque three.
Gelatin empty capsules has a good taste-masking effect, good bioavailability, beautiful and easy to swallow, etc., is a wide selection of pharmaceutical excipients, disintegration and absorption occurs primarily in the stomach.
Gelatin empty capsules models can be divided into 00 # 0 # 0 # lengthened, # 1, # 1 lengthened, # 2, # 3, # 4, and other specifications, to meet the needs of a variety of drug loading filling amount.
Our gelatin capsule products can provide a variety of printing mode printing hoop and axial printing, color printing, etc., you can render your unique custom look.Consistent with and above the "People's Republic of China Pharmacopoeia" 2015 version of the standard four.