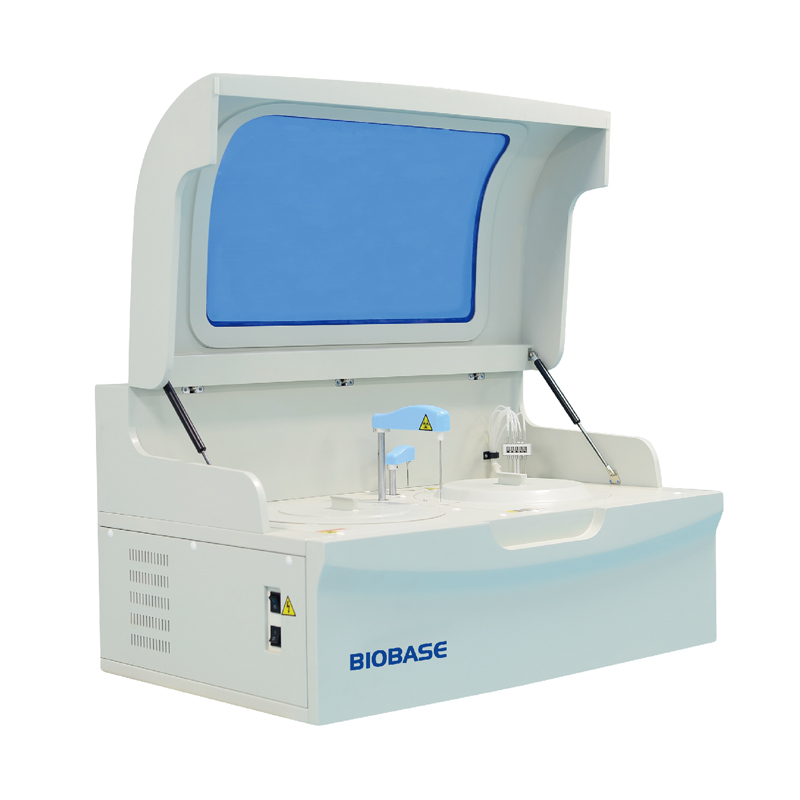
- Type:
- Blood Analysis System
- Brand Name:
- BIOBASE
- Model Number:
- BK-200
- Place of Origin:
- Shandong, China
- Instrument classification:
- Class I
- Analysis method:
- End point, two points, kinetic method, turbidimetry
- Wavelength range:
- 340, 405, 510, 546, 578, 600, 660, 700
- Receiver:
- 8 imported photo receivers
- Sample volume:
- 3 ul-50ul
- Cuvette:
- 120 independent reaction cuvettes
- Calibration:
- one-point, multipoint
- Test/H:
- 200
- Shipping Dimensions, (W x D x H):
- 1100*600*500mm
- Certification:
- CE, ISO9001, ISO14001, ISO13485, FDA
- Supply Ability:
- 1000 Set/Sets per Year
- Packaging Details
- wooden case
- Port
- Qingdao
FDA certificated Bench-top chemistry laboratory equipment and automatic chemistry analyzer made in China

1. Benchtop type,Fully automatic, Discrete, Random access
2.200 T/H,46 sample position and 59 reusable cuvettes.
3. 90 refrigerated reagent tray (R1:45 R2:45), 24 hour working.
4. Reversed optics and imported photo sensor, ensuring the precision
of detection results.
5. Precision dispense pumps assures the accuracy ofadding sample volmue,
sample volume step by 0.1ul, reagent volume step by 1ul.
Model | BIOBASE-SAPPHIRE |
Dimensions (W x D x H) | 900*500*400mm |
Analysis method | End point, two points, kinetic method, turbidimetry |
Wavelength range | 340, 405, 510, 546, 578, 600, 660, 700 (three of them can be defined by the users), adopting reversed optics |
Reagent volume | R1: 25-300ul; R2: 10-150ul(step by 1ul), support reagent from different countries |
Receiver | 8 imported photo receivers |
Sample volume | 3 ul-50ul (step by 0.1ul), high precision injector (dilution vessel) is prepared |
Cuvette | 120 independent reaction cuvettes(optical path length 6mm) |
Calibration | one-point, multipoint |
Test/H | 200 |
Shipping Dimensions, | 1100*600*500mm |
Software | Windows 2000 professional or windows XP. Have plenty of functions of inquiries, convenient to use |
Printout | user can modify the printing format by his own |
Electrical | AC220V + 22V 50Hz + 1Hz |
Certification | CE, ISO9001, ISO14001, ISO13485, FDA |




Founded in 1999, having 1400 employees (till September, 2016), BIOBASE Group is a new high-tech enterprise focusing on development, production and management of scientific equipment, In Vitro Diagnosis (IVD) instrument and reagent.

BIOBASE Group is specialized in products of 8 areas include medical diagnosis, biosafety protection, disinfection and sterilization, water purification system, infant care products, cold chain products, software products, clean room project.

Gathering up a professional team of experts, scholars as well as other scientific research personnels, our company has obtained patents and expanded marketing for multiple products such as the self-developed Biochemistry analyzer, 107 kinds of Reagents, Automatic Elisa processor, Medical sterilization instrument and Biosafety protection equipment.
BIOBASE Group has certificates include ISO9001, ISO13485, ISO 14001, SGS, CE, NSF, EN, FDA, etc.



Contact