- Type:
- Pressure Steam Sterilization Equipments
- Brand Name:
- BIOBASE
- Model Number:
- BKM-Z18B
- Place of Origin:
- Shandong, China
- Instrument classification:
- Class II
- Sterilization Temp.:
- 121℃, 134℃
- Sterilization Class:
- CLASS B
- Dry System:
- Vacuum drying system
- Capacity:
- 18L
- Special Program:
- HIV, HBV long-sterilization program
- certificate:
- CE, ISO
- Material:
- Stainless Steel SUS304
- Voltage:
- AC110V 60Hz/AC220V 50Hz
- Supply Ability:
- 500 Unit/Units per Month
- Packaging Details
- wooden package
- Port
- Qingdao
- Picture Example:
![package-img]()
![package-img]()
- Lead Time:
Quantity(Units) 1 - 1 >1 Est. Time(days) 15 To be negotiated
18L CE certificate LCD display usb interface built in mini printer Class B dental autoclave

BKM-ZB series sterilizer is an automatic high temperature and pressure rapid sterilizer which works with steam as medium.
It can be widely used in department of stomatology and ophthalmology, operating room, supply room, dialysis room and other medical institutions. It is unpacked items, solid instruments, dental hand pieces, endoscopes, implantable instruments, dressing fabric and rubber tubes, etc.
Autoclave Microbiology Features:
1. Build-in open type water tank
The sterilizer adopts easy-clean open type water tank which can support repeated program running if fully injected
with water.
2. High-efficiency ultimate vacuum
The sterilizer adopts high-efficiency low noise vacuum system which has excellent effects.
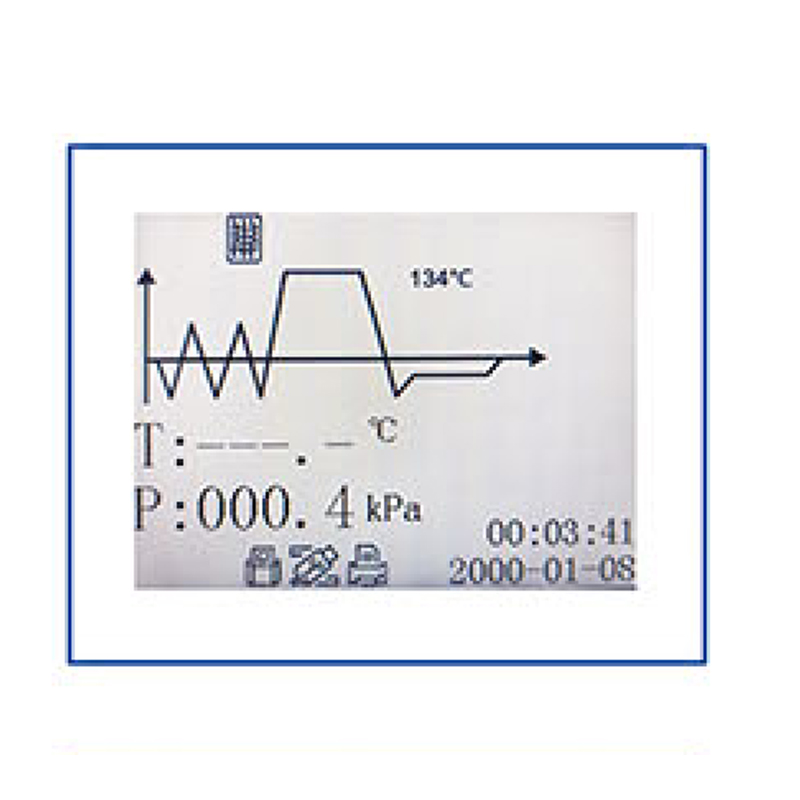
3. Large LCD display for BKM-Z18/24B
The LCD screen can display temperature, pressure, time, operating status, failure warning and other information.
It is convenient for customers to observe the sterilizer running status.
4. Multiple program types
The system has various programs that include: packed items, unpacked items, custom program,
rapid program, BD testing program, vacuum testing program, preheat program and drying program.
5. Standard USB port for BKM-Z18/24B
Users can store the sterilization data with USB disk.
6. Optional mini printer can be attached to record the process of sterilization.
Autoclave Technical Parameter
Model | BKM-Z16B | BKM-Z18B | BKM-Z24B |
Capacity | 16L | 18L | 24L |
Chamber Size(mm) | φ230*360 | φ247*360 | φ247*470 |
Sterilization Class | Class B (according toGB0646) | ||
Sterilization Temp. | 121℃,134℃ | ||
Special Program | HIV, HBV long-sterilization program | / | |
Drying System | Vacuum drying system | ||
Display | LED display | LCD display | |
Testing System | B&D Test Vacuum Test Helix Test | ||
Control Precision | Temperature: 1℃ Pressure: 0.1bar | ||
Sterilization Data | BKM-Z16B:Printer(optional) BKM-Z18B/BKM-Z24B:USB(standard) and printer(optional) | ||
Safety System | Hand lock door Pressure lock system Relief valve in case of over pressure Pressure or temperature over load protection Alarm for system failure, finish reminding, water level warning | ||
Water Supply System | Build-in water tank easy to clean | ||
WaterTankCapacity | 2L | 4L | |
Water Consumption | 0.4L in one cycle | 0.16L~0.18L in one cycle | |
Tray Holder | 3 pcs SS trays on SS shelf | ||
Chamber | SUS304 Max working pressure:2.3bar Min working pressure: -0.9bar Designtemperature: 140℃ | ||
Ambient Temp. | 5~40℃ | ||
Noise | <50dB | ||
Consumption | 1800W | 1950W | 1950W |
Power Supply | 110/220V±10%,50/60Hz | ||
External Size(W*D*H)mm | 445*550*395 | 495*600*410 | 495*700*410 |
Packing Size(W*D*H)mm | 670*550*500 | 610*810*590 | 610*810*590 |
Gross Weight(kg) | 47 | 63 | 65 |
Note:It is applicable to the altitude below 3,500m
Founded in 1999, BIOBASE is a world leading high-tech enterprise focusing on Research & Development, manufacturing and management of laboratory and pharmaceutical equipment. Products have served for clients in more than 100 nations.
BIOBASE provides more than 150 different types of product, including Fully Automated Biochemistry Analyzers, Automated Elisa Processors, Biological Safety Cabinets, Fume Hood, Laminar Flow Cabinet, Autoclaves, Refrigerators, Freezers, Incubators, Water Purifiers and etc. The company has been certified by international standards such as ISO 9001, ISO 13485 and ISO 14001. Various products have obtained international certifications like CE, MET, ETL, and have been registered in FDA, RCM, etc.

With more than 2000 employees and 50000 sqm production base, BIOBASE remains dedicated to deliver innovative solutions for the users with a strong R&D team formed by post-graduates and doctors. The service team is adequately qualified to offer training and service on the installation and maintenance for the full range of products. Engineers are experienced on hands-on design, manufacturing, testing and troubleshooting.
If you want to learn more about our company, please click here to see more.


Robert Wang