Authors and their institutions
Tong Wu , Jiajia Xue , Haoxuan Li, Chunlei Zhu, Xiumei Mo, and Younan Xia
The Wallace H. Coulter Department of Biomedical Engineering, Georgia Institute of Technology and Emory University, Atlanta, Georgia 30332, United States
State Key Lab for Modification of Chemical Fibers and Polymer Materials, College of Chemistry, Chemical Engineering and Biotechnology, Donghua University, Shanghai 201620, P. R. China
School of Chemistry and Biochemistry, School of Chemical and Biomolecular Engineering, Georgia Institute of Technology, Atlanta, Georgia 30332, United States
Periodical
ACS Appl.Mater.Interfaces
Impact Factor
7.504
Article info
Received 3 January 2018
Date accepted 8 February 2018
Published online 8 February 2018
Abstract
Scaffolds functionalized with circular gradients of active proteins are attractive for tissue regeneration because of their enhanced capability to accelerate cell migration and/or promote neurite extension in a radial fashion. Here, we report a general method for generating circular gradients of active proteins on scaffolds composed of radially aligned nanofibers. In a typical process, the scaffold, with its central portion raised using a copper wire to take a cone shape, was placed in a container (upright or up-side-down), followed by dropwise addition of bovine serum albumin (BSA) solution into the container. As such, a circular gradient of BSA was generated along each nanofiber. The bare regions uncovered by BSA were then filled with an active protein of interest. In demonstrating their potential applications, we used different model systems to examine the effects of two types of protein gradients. While the gradient of laminin and epidermal growth factor accelerated the migration of fibroblasts and keratinocytes, respectively, from the periphery toward the center of the scaffold, the gradient of nerve growth factor promoted the radial extension of neurites from the embryonic chick dorsal root ganglion. This method for generating circular gradients of active proteins can be readily extended to different types of scaffolds to suit wound closure and related applications that involve cell migration and/or neurite extension in a radial fashion.
Results and discussion
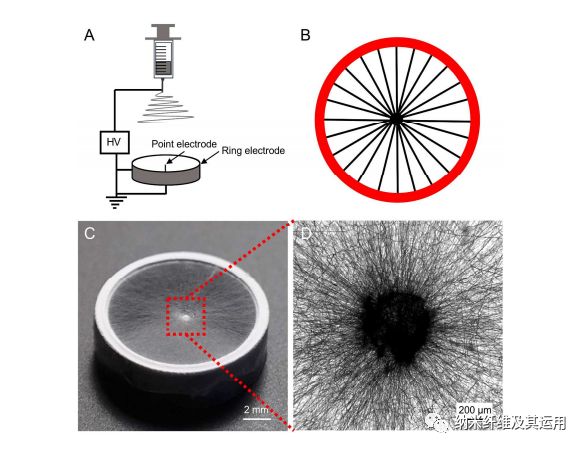
Figure 1. (A) Schematic of the electrospinning setup used for generating a scaffold consisted of radially aligned PCL nanofibers. (B) Schematic showing the nanofibers aligned across the point and ring electrodes. (C) Photograph showing a typical sample of radially aligned PCL nanofibers. (D) Optical micrograph captured from the central portion of the scaffold.
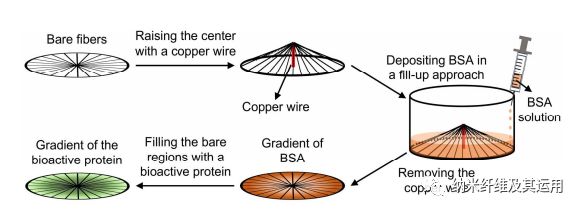
Figure 2. Schematic for generating a circular gradient of active protein along radially aligned nanofibers. A plasma-treated nanofiber scaffold, whose center is raised by 4 mm with a copper wire, is placed in the well of a 24-well plate. A gradient of BSA is then created by pumping 0.1% BSA solution into the well at a rate of 1 mL/h. The bare regions left by BSA are then filled with an active protein to generate a gradient that runs countercurrent to the BSA gradient. In this way, a radial gradient increasing from the periphery to the center is generated. Similarly, an opposite gradient increasing from the center towards the periphery can be produced using the same approach except that the center-raised scaffold is placed in the well up-side-down.
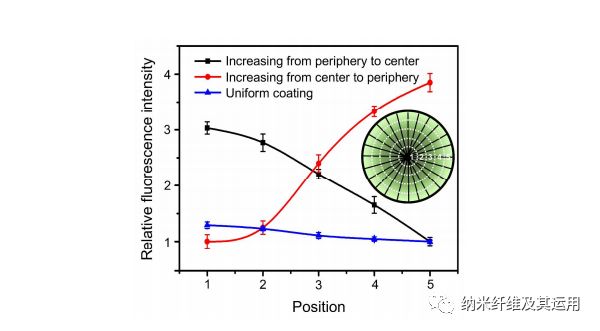
Figure 3. Relative fluorescence intensity showing a graded or uniform coating of FITC-BSA along the radially aligned nanofibers generated through BSA blocking. The inset shows a schematic of the corresponding positions along the radial direction.
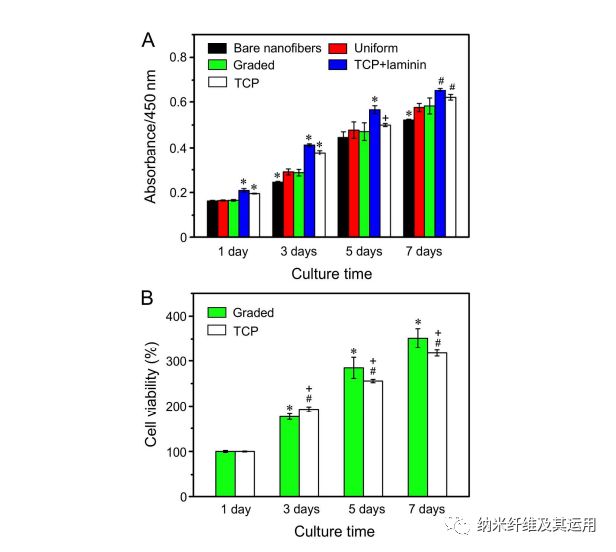
Figure 4. (A) The proliferation of NIH-3T3 fibroblasts on the different types of nanofiber scaffolds tested by CCK-8 assay. *P < 0.05 relative to the other groups at the same culture time; #P < 0.05 relative to the scaffolds coated with graded and uniform laminin, and bare nanofibers; +P < 0.05 as compared with that on bare nanofibers. (B) The viabilities of cells cultured on the scaffolds covered with a gradient of laminin and on TCP at different incubation times. *P < 0.05 as compared with that on scaffolds coated with graded laminin at 1 day; #P < 0.05 as compared with that on TCP at 1 day; +P < 0.05 as compared with that on scaffolds coated with graded laminin at the same time point.
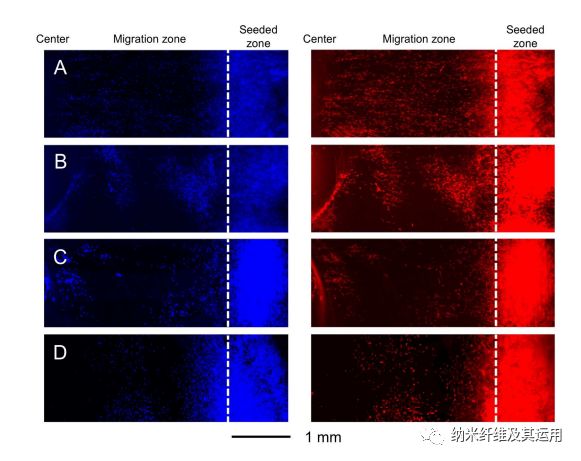
Figure 5. Fluorescence micrographs showing the migration of NIH-3T3 fibroblasts on radially aligned nanofibers covered with (A) a graded and (B) uniform coating of laminin, respectively, (C) bare radially aligned nanofibers, and (D) laminin-coated TCP after culture for 3 days. The cells nuclei were stained with DAPI (blue) and the actin cytoskeleton was stained with Alexa Fluor 555 phalloidin (red). The dashed lines indicate the border of cell seeding.
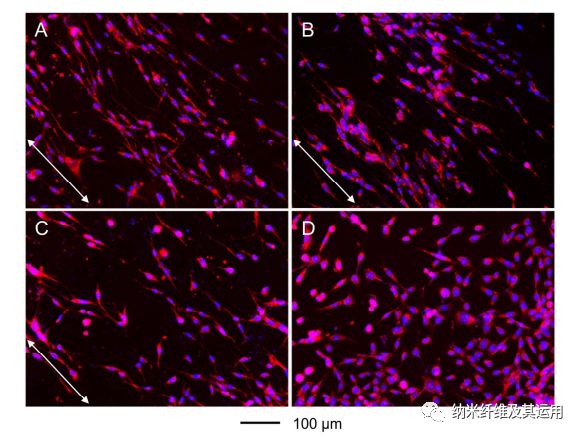
Figure 6. Fluorescence micrographs showing the morphology and alignment of NIH-3T3 fibroblasts cultured on radially aligned nanofibers covered with (A) a graded and (B) uniform coating of laminin, respectively, (C) bare radially aligned nanofibers, and (D) laminin-coated TCP. The images were captured from the migration zones. The cells nuclei were stained with DAPI (blue) and the actin cytoskeleton was stained with Alexa Fluor 555 phalloidin (red). The arrows indicate the direction of nanofiber alignment.
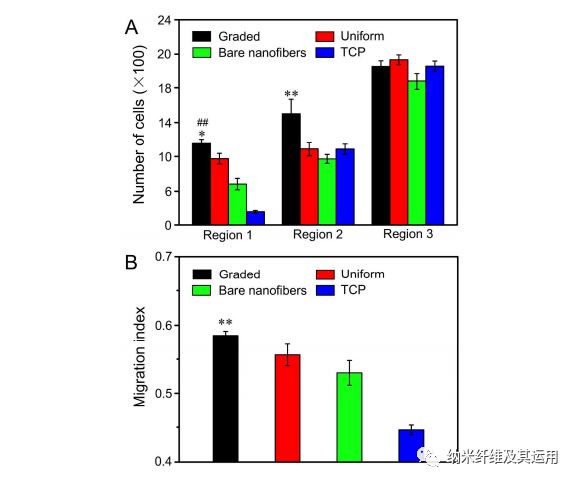
Figure 7. (A) The number of cells in different regions of the migration zone on radially aligned nanofibers covered with a graded or uniform coating of laminin, bare nanofibers, and laminin-coated TCP after 3 days of culture. *P < 0.05 as compared with that on scaffolds covered with a uniform coating of laminin; ##P < 0.01 relative to the scaffolds made of bare nanofibers and laminin-coated TCP; **P < 0.01 relative to the scaffolds covered with a uniform coating of laminin, scaffolds made of bare nanofibers, and laminin-coated TCP. (B) Migration index calculated from the numbers of migrated cells in different regions. **P < 0.01 when compared with those of cells cultured on scaffolds covered with a uniform coating of laminin, scaffolds made of bare nanofibers, and laminin-coated TCP.
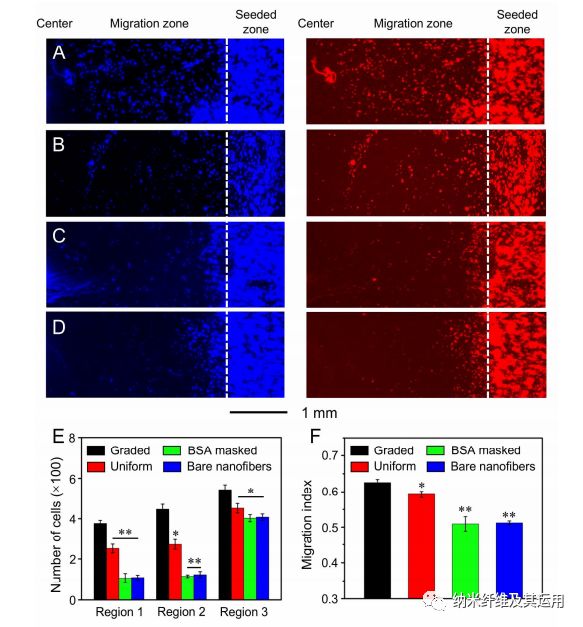
Figure 8. Fluorescence micrographs showing the migration of keratinocytes on radially aligned nanofibers covered with (A) a graded and (B) uniform coating of EGF, respectively, (C) radially aligned nanofibers masked by a gradient of BSA, and (D) bare radially aligned nanofibers after culture for 3 days. The cells nuclei were stained with DAPI (blue) and the actin cytoskeleton was stained with Alexa Fluor 555 phalloidin (red). The dashed lines indicate the border of cell seeding. (E) The number of cells in different regions of the migration zone on different scaffolds after 3 days of culture. (F) Migration index calculated from the numbers of migrated cells in different regions. *P < 0.05 and **P < 0.01 when compared with that of cells cultured on scaffolds coated with graded EGF.
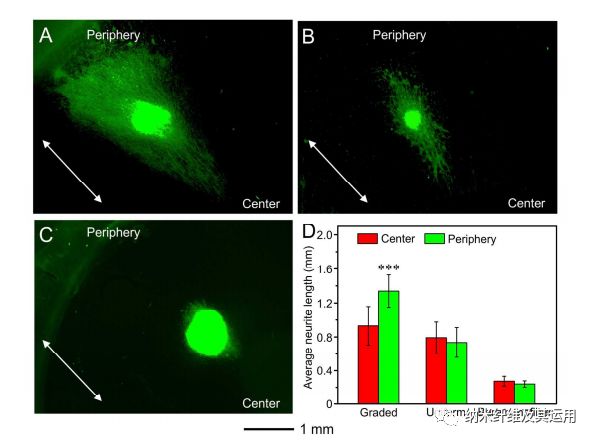
Figure 9. Fluorescence micrographs of the typical neurite fields extending from DRG bodies when cultured on radially aligned nanofibers coated with (A) a graded and (B) uniform layer of NGF, respectively, and (C) bare radially aligned nanofibers for 6 days. The neurites were stained with Tuj1 marker (green). The arrows indicate the direction of nanofiber alignment. (D) Analyses of the average lengths of the neurites outgrowing from the chick DRG bodies cultured on different nanofiber scaffolds. ***P < 0.001 as compared with that extending from DRG bodies towards the center. No significant difference was found between either side of the DRG bodies on scaffolds covered with a uniform coating of NGF and made of bare nanofibers.
Conclusions
In summary, we have demonstrated a simple and general strategy for generating circular gradients of active proteins on radially aligned nanofibers by firstly creating a graded mask of BSA. By constantly increasing the volume of BSA solution introduced into a container with a center-raised nanofiber scaffold placed in the upright or up-side-down configuration, a gradient of BSA can be generated along the radially aligned nanofibers. Then, the bare regions left behind by the BSA are filled with an active protein of interest to generate a circular gradient of the active protein that runs countercurrent to BSA. This class of scaffolds is able to present both a topographic cue in radial alignment and a biochemical cue in protein gradient towards the center or the periphery, which can be readily adapted for various types of active proteins and polymer scaffolds. As a demonstration for the potential applications in tissue engineering, a circular gradient of laminin, EGF, or NGF was generated on radially aligned PCL nanofibers along the nanofiber orientation. We showed that the migration of fibroblasts or keratinocytes towards the center of the nanofiber scaffold could be accelerated with the presence of a laminin or EGF gradient along the radial alignment. For the radially aligned nanofibers coated with a gradient of NGF, DRG neurites extended along the radial alignment of nanofibers, and the neurites extending towards the increase in NGF content were significantly longer by 1.44-fold than those extending in the opposite direction. Taken together, these studies provide valuable information for designing biochemically graded scaffolds integrated with a radially aligned topographical cue for potential use in wound closure and related applications requiring cell migration and/or neurite extension in a radial pattern.
Statement:
1. Paper link: http://www.qingzitech.com/inno_detail/newsId=162.html
2. This article is for academic exchange only, welcome to share and reprint by scholars; if there is any infringement, please contact us to make modification.

