Recently, by the Foshan Lepton Precision M&C Tech Co., Ltd customer, International Graduate School of Tsinghua University in Shenzhen, Shengli Mi and Wei Sun Group, have made great progress in the field of corneal stromal regeneration, relevant results were published in Nature Communications Journal on March 18, 2020, titled " Fiber reinforced GelMA hydrogel to induce the regeneration of corneal stroma”.
The cornea locates at the outermost surface of the eye, and it plays an essential role in the visual system; it supplies two-thirds’ of optical power, protects the intraocular structures and tissues, and refracts light onto the retina. Injuries, bacterial and viral infections, and congenital and degenerative conditions may damage the function of the cornea, making corneal damage the second leading cause of blindness. More than 10 million individuals with diverse corneal disorders worldwide are reported every year. Penetrating keratoplasty is the most commonly used grafting procedure to improve visual impairment from severe corneal diseases due to the high short-term success; however, the availability of donor corneal tissue cannot meet the global requirements. Artificial keratoprostheses, animal decellularized corneal tissue (e.g., porcine cornea), and human amniotic membrane have also been used for the treatment of corneal disorders because of the shortage of donor cornea; however, these methods do not have high success ratios or approved use in tissue transplantation, despite that some methods are in current clinical practice. For the reason above, it is essential and urgent to develop therapeutic alternatives to corneal transplantation, including cell-based therapy and bioengineered constructs.
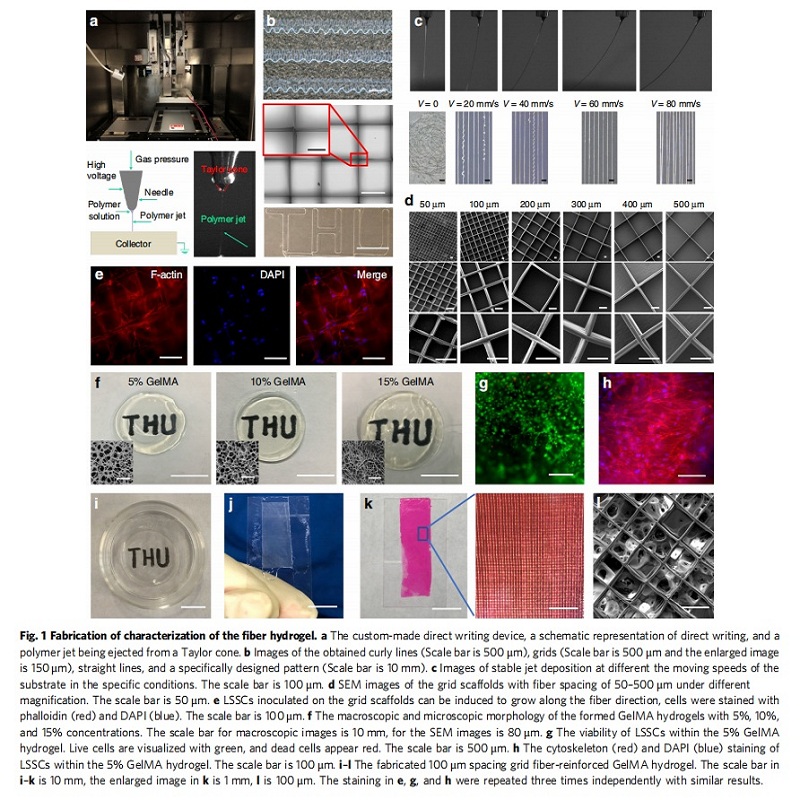
Regeneration of corneal stroma has always been a challenge due to its sophisticated structure and keratocyte-fibroblast transformation. In this study, they fabricate grid poly (ε-caprolactone)-poly (ethylene glycol) microfibrous scaffold and infuse the scaffold with gelatin methacrylate (GelMA) hydrogel to obtain a 3D fiber hydrogel construct; the fiber spacing is adjusted to fabricate optimal construct that simulates the stromal structure with properties most similar to the native cornea. The topological structure (3D fiber hydrogel, 3D GelMA hydrogel, and 2 D culture dish) and chemical factors (serum, ascorbic acid, insulin, and β-FGF) are examined to study their effects on the differentiation of limbal stromal stem cells to keratocytes or fibroblasts and the phenotype maintenance, in vitro and in vivo tissue regeneration. The results demonstrate that fiber hydrogel and serum-free media synergize to provide an optimal environment for the maintenance of keratocyte phenotype and the regeneration of damaged corneal stroma.
Fabrication of PECL microfibrous scaffold. The direct writing device used to fabricate microfibers was custom-made by the Foshan Qingzinano Corporation primarily using a pneumatic extrusion system with a stainless syringe, a precise X–Y translational stage with a collector plate (conductive glass) to execute the predesigned patterning route (e.g., straight, grid, curly, and arbitrary), and a high voltage power. A high-speed camera (HuaGuDongLi, SHL-200WS) was used to observe the morphology and motion of the jet. One gram of synthesized PECL copolymer was heated to 105 °C for 10 min and extruded at an air pressure of 20 kPa. With the 3 mm distance between the syringe needle (30G stainless) and the collector plate, and 3.6 kV electrostatic voltage, a stable PECL jet was obtained. The jet and fiber morphology under the stage velocity of 0, 20, 40, 60 and 80 mm/s were determined. The grid fibrous scaffolds were fabricated with a fiber spacing of 50, 100, 200, 300, 400, and 500 μm, and a scaffold height of 100 μm to mimic the
orthogonally aligned stromal layer. The morphology of the writing fibers was imaged using a microscope with a super-wide depth of field (YEYENCE, VHX-6000) and Scanning Electron Microscope (SEM, Phenom XL) at 10 kV (Samples were pre-coated with a 20-nm-thick layer of platinum). The average fiber diameters were digitized and analyzed by Image Pro Plus (IPP) software. LSSCs inoculated on the grid fibrous scaffold were observed by cytoskeleton/nuclei staining.
Links to papers: https://doi.org/10.1038/s41467-020-14887-9